- Contact Person : Ms. Wong Jennifer
- Company Name : Fuzhou Changgeng Medical Devices Co., Ltd.
- Tel : 86-591-22060591
- Fax : 86-591-22060593
- Address : Fujian,Fuzhou,No.1, Southern District, Minhou ETDZ 2#, Ganzhe Street,Fuzhou,China
- Country/Region : China
- Zip : 350100
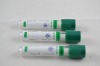
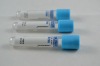
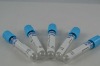
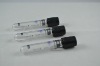
Related Product Searches:Platic Vacuum EDTA K2 tube,High Quality,vacuum blood collection tube, EDTA2K tube
Description:
Material | PET or Glass | |
Draw volume | PET: 13*75MM,4ml 13*100MM,6ml 16*100MM,10ml | Glass: 12*75MM 12*100MM 16*100MM |
Cap color | Lavendar | |
Closure type | Safety | |
package | 100pcs/tray, 1000pcs/carton | |
measurement | Carton of 13*75MM: 470*300*195MM Carton of 13*100MM: 585*300*195MM Carton of 16*100MM: 580*330*215MM | |
Shelf life | 1 YEAR | |
others | Storage in room temperature. Single use! | |
Brand name | CG | |
Payment terms | T/T | |
FOB port | Fuzhou | |
Place of origin | Fuzhou, China | |
Contract manufacturing | OEM service offered | |
Application:
This vacuum tube adopts EDTA2K or EDTA3K as an anticoagulant. They are widely used in clinical blood tests and a variety of blood cell analysis.
( function(){ var aBigImageSrc = [ 'http://i01.i.aliimg.com/photo/v0/549070807_1/Platic_Vacuum_EDTA_K2_tube.jpg' , 'http://i01.i.aliimg.com/photo/v0/549070807_2/Platic_Vacuum_EDTA_K2_tube.jpg' , 'http://i01.i.aliimg.com/photo/v0/549070807_3/Platic_Vacuum_EDTA_K2_tube.jpg' , 'http://i01.i.aliimg.com/photo/v0/549070807_4/Platic_Vacuum_EDTA_K2_tube.jpg' , 'http://i01.i.aliimg.com/photo/v0/549070807_5/Platic_Vacuum_EDTA_K2_tube.jpg' , 'http://i01.i.aliimg.com/photo/v0/549070807_6/Platic_Vacuum_EDTA_K2_tube.jpg' ]; var oProductShow = new AE.run.minisite.productShow(); oProductShow.init( { 'aImgSrc': aBigImageSrc } ); } )(); Platic Vacuum EDTA K2 tube